Unlocking the Potential of Clinical Trials: A Comprehensive Guide to Designs

5 out of 5
| Language | : | English |
| File size | : | 3891 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 222 pages |
Clinical trials are the cornerstone of medical research, providing crucial evidence for the development of new treatments and therapies. The design of these trials plays a pivotal role in ensuring their effectiveness and reliability. This comprehensive guide delves into the principles of clinical trial design, providing a deep understanding of the different types of designs and their applications. Whether you're a novice or an experienced researcher, this guide will equip you with essential knowledge to navigate the complexities of clinical trial design.
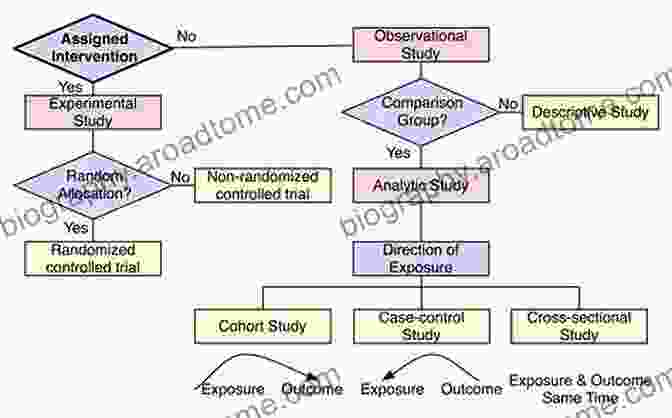
Types of Clinical Trials
Clinical trials come in a variety of forms, each suited to specific research objectives. Here are some of the most common types:
- Observational studies: These studies observe participants in their natural settings without actively intervening. They are primarily used to identify risk factors and explore associations between variables.
- Interventional studies: These studies actively assign participants to different treatment groups to evaluate the effectiveness and safety of new treatments.
- Randomized controlled trials (RCTs): RCTs are the gold standard for clinical trials. Participants are randomly assigned to different treatment groups, ensuring an unbiased comparison of outcomes.
- Cohort studies: These studies follow a group of individuals over time to observe the development of a disease or condition.
- Case-control studies: These studies compare individuals with a disease or condition to individuals without the condition to identify potential risk factors.
Randomization and Blinding
Randomization and blinding are essential principles of clinical trial design that help to reduce bias and enhance the validity of results.
- Randomization: Randomly assigning participants to different treatment groups ensures that each group is representative of the target population and reduces the influence of confounding factors.
- Blinding: Keeping participants and researchers unaware of which treatment group they are in helps to prevent bias and ensures that subjective factors do not influence the results.
Control Groups
Control groups play a crucial role in clinical trials by providing a baseline for comparison of treatment effects. Different types of control groups can be used, depending on the research question.
- Placebo groups: These groups receive a placebo, an inert substance that resembles the active treatment.
- Active control groups: These groups receive a standard or current treatment for the condition being studied.
- No treatment groups: These groups do not receive any treatment or intervention.
Study Populations
The choice of study population is critical for ensuring the generalizability and applicability of clinical trial results.
- Target population: The population to which the results of the trial are intended to apply.
- Inclusion criteria: Characteristics that participants must meet to be eligible for the trial.
- Exclusion criteria: Characteristics that make individuals ineligible for the trial.
Data Collection
Data collection is a crucial aspect of clinical trials, as it provides the raw material for analysis and interpretation.
- Study protocols: These documents outline the procedures and methods used in the trial, ensuring consistency and reducing bias.
- Case report forms (CRFs): These forms capture patient data and observations during the trial.
- Electronic data capture (EDC) systems: These systems automate data collection, reducing errors and increasing efficiency.
Understanding the principles of clinical trial design is paramount for researchers and healthcare professionals involved in the development and evaluation of new treatments. By carefully considering the type of trial, randomization, blinding, control groups, study population, and data collection methods, researchers can ensure the validity and reliability of their findings. This comprehensive guide provides a solid foundation for anyone looking to delve into the fascinating world of clinical trial design.
Additional Resources
- The CONSORT Statement: https://www.consort-statement.org/
- The Cochrane Handbook for Systematic Reviews of Interventions: https://handbook.cochrane.org/
- The Medical Research Council's (MRC) "Epidemiology for a New Century" Report: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2706942/
5 out of 5
| Language | : | English |
| File size | : | 3891 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 222 pages |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia 21 Exercises
21 Exercises Hannah J Stolze
Hannah J Stolze Tiffany Field
Tiffany Field Kathy Seaman Shaw
Kathy Seaman Shaw Molly Coxe
Molly Coxe Arthur Rothstein
Arthur Rothstein A J Demas
A J Demas Lita Epstein
Lita Epstein Vaudine England
Vaudine England B Zorina Khan
B Zorina Khan Albert Bandura
Albert Bandura D S Malik
D S Malik S L Bridle
S L Bridle Jackie Notman
Jackie Notman Scott Millett
Scott Millett Amy Traynor
Amy Traynor Tan France
Tan France Jeremy Raymond
Jeremy Raymond David Owen
David Owen David Mixson
David Mixson
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!
 Matthew WardFollow ·14.3k
Matthew WardFollow ·14.3k Manuel ButlerFollow ·8.5k
Manuel ButlerFollow ·8.5k Brian BellFollow ·18.8k
Brian BellFollow ·18.8k Howard BlairFollow ·17.5k
Howard BlairFollow ·17.5k Julian PowellFollow ·7.6k
Julian PowellFollow ·7.6k Randy HayesFollow ·18.4k
Randy HayesFollow ·18.4k Evan SimmonsFollow ·2.7k
Evan SimmonsFollow ·2.7k Grant HayesFollow ·7.8k
Grant HayesFollow ·7.8k
 Ashton Reed
Ashton ReedUnveiling the Silent Pandemic: Bacterial Infections and...
Bacterial infections represent...

 Brent Foster
Brent FosterFinally, Outcome Measurement Strategies Anyone Can...
In today's...

 Brett Simmons
Brett SimmonsUnlocking the Secrets to Entrepreneurial Excellence:...
Empowering...

 Eugene Powell
Eugene PowellOur Search For Uncle Kev: An Unforgettable Journey...
Prepare to be captivated by...
5 out of 5
| Language | : | English |
| File size | : | 3891 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 222 pages |